What it does
The capabilities of SPARTA are meeting the unmet needs of the industry

Would you invest in a product with a 95% failure rate?
This is the case in pharmaceutical research and development, where only around 1 in 20 drugs pass clinical trial. Each failure in this late stage can cost upwards of $30 million, amounting to catastrophic wastage of time, effort, and money.
What if we could more accurately screen drugs earlier, and increase the hit-rate of successful trials?
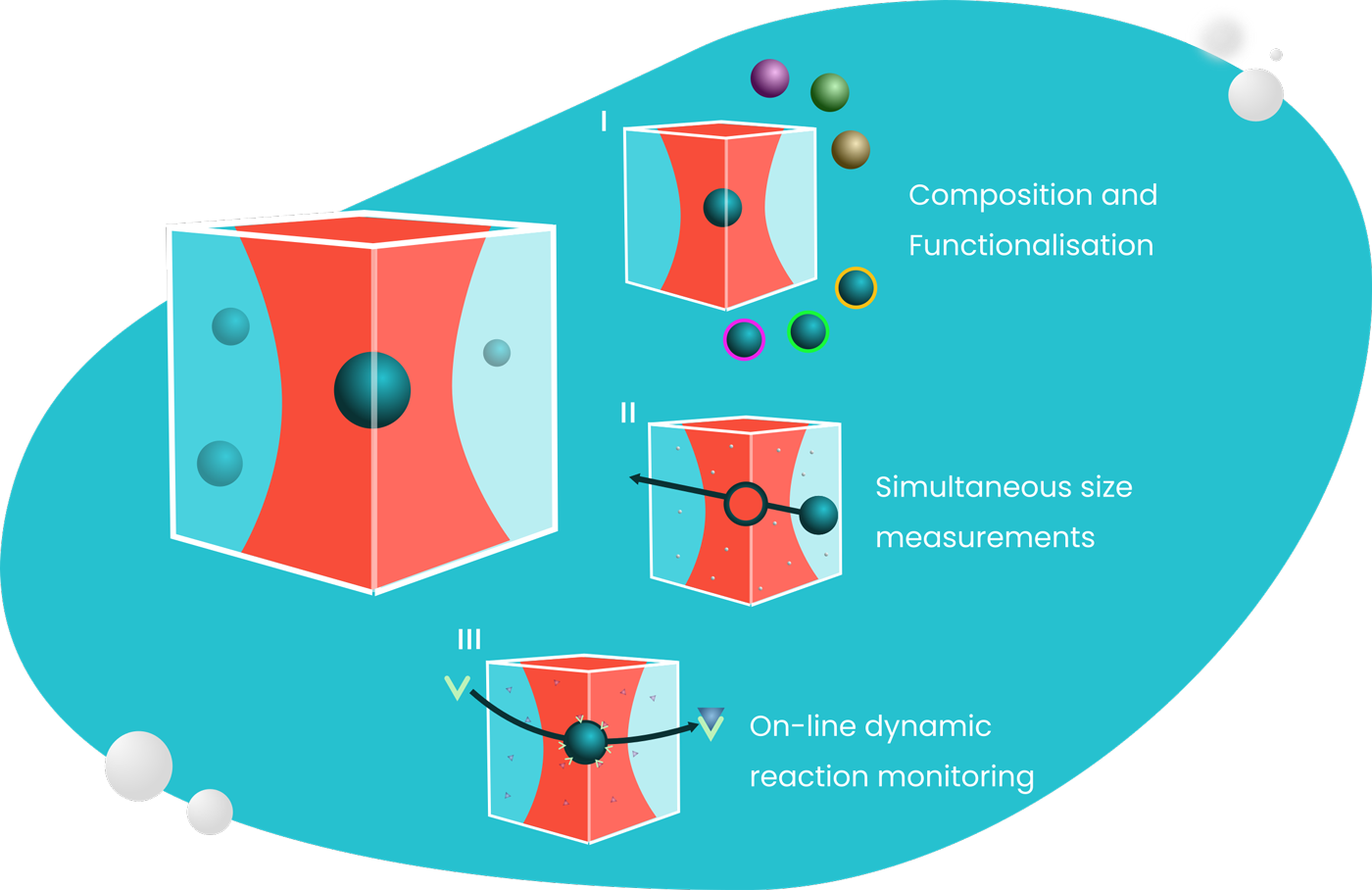
One method to achieve this for nanoformulations (gene therapy, vaccines, antimicrobials, chemotherapy) is the laboratory analysis of drug formulations at the nanoscale – gleaning information from molecules and materials which are about a million times smaller than this full stop.
Current technologies for composition analysis rely on bulk methods; processing large collections of nanoparticles together and assessing their properties at a population level. These methods include nanoflow cytometry, polymerase chain reaction (PCR) and liquid chromatography mass spectroscopy (LC-MS).
However, the complexity of novel nanoformulations means that there is higher chance of variation on an intrabatch level, which bulk techniques cannot address.
The capabilities of SPARTA are meeting the unmet needs of the industry


Prof. of Biomedical Materials and Regenerative Med. & Research Director for Biomedical Material Sciences at Imperial College London

Experienced postdoctoral research associate in the field of biomaterials and characterisation, and co-inventor of SPARTA®

Founder and Chief Executive of Avacta Group plc with extensive experience building early stage businesses within Avacta Group during fifteen years as a public company CEO

Translational research manager with experience in funding development and IP management